Children in research: Many parents willing if risk of harm is small


Children in research: Many parents willing if risk of harm is small
Parents and physicians want medicines for children that are safe and effective. Research involving children is critical to identifying medicines that work best for children, in order for the Food and Drug Administration (FDA) to give approval for those medicines for use in children.
There are many challenges to doing research involving children. Perhaps most important is that parents must be convinced that their children will be safe in the research studies. Additionally, parents must understand that having children in research is essential to discover the safest and most effective doses of medicines for children. Figuring out what parents think of child participation in research, and reasons to participate or not to participate, was the goal of this poll.
Participation of Children in Research
In December 2007, the C.S. Mott Children’s Hospital National Poll on Children’s Health asked adults if they themselves or their children have ever participated in clinical research:
- 10% of adults have participated in research
- 4% of parents have had their children participate in research.
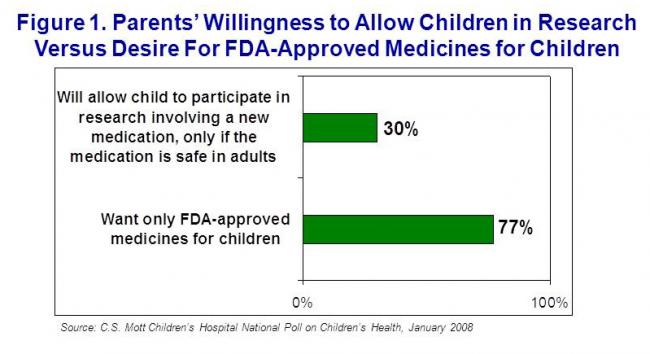
Parents were asked if they were willing to allow their children to participate in research involving a new medication, after the medication had been shown to be safe in adults. 30% of parents said they would allow their children to participate. This is substantially less than the proportion of parents (77%) who want their doctor to prescribe only medicines that are FDA-approved for use in children, which was measured and previously reported from the same poll (NPCH, Vol. 3, Issue 4, April 2008) (Figure 1).
One-quarter of parents said they would allow their children to participate in research as a healthy volunteer, if the risk were small. About one-third of parents were willing to allow their child to participate only if he/ she had the disease being studied. In both of these situations a substantial number of parents were unsure about their children participating in research and a minority of parents would not allow their children to participate (Figure 2).
Parents’ Reasons For Children To Participate
Researchers may find it helpful to understand why parents would want their children to participate in research. Reasons to participate included:
- If the risk of harm were small, 42%
- If the disease being studied ran in their family, 32%
- If their doctor encouraged participation, 30%
- If the research would help other children, 27%
- If the child received payment, 17%.
Parents’ Reasons For Children Not to Participate
Parents may not want their children to participate in research for several reasons. Parents were asked to indicate which reasons would lead them to not allow their children to participate in research. Reasons not to participate included:
- There is too high a chance for harm, 73%
- I do not want my child to be a “guinea pig”, 60%
- It is not appropriate for children to participate, 41%
- If my child’s doctor was not directly involved, 38%
- If the disease did not affect my child, 36%.
Majority of Parents Have Not Been Asked About Child Participation in Research
Of the parents whose children have not participated in research, only 4% have been asked to participate and 92% have never been asked to participate (Figure 3).
Highlights
- 77% of parents want only FDA-approved medicines for their children, but only 30% say they will allow their children to participate in research.
- Many parents are willing to consider children's participation in research if the risk of harm is small.
- 92% of parents have never been asked to have their children participate in research.
Implications
Medical research is critical to the process of making sure prescription drugs and vaccines are as safe and effective as possible for children. Because most parents want only FDA-approved medicines for their children, we know that parents value the information that comes from research. However, there is a large gap between the proportion of parents who want safe medicines and the proportion who are willing to have their children participate in the medical research that would produce information about medicine safety. That gap ultimately leaves parents with less information about medicines than they themselves want.
This poll also indicates that there is the potential for many more children to participate in research than have done so before. Only about 4% of parents have had a child participate in research, and the vast majority have never been asked. But about one-third of parents say they would consider allowing their children to participate if a drug had already been proven safe in adults, or if their children had a particular disease being studied.
These findings are exciting for researchers and for groups who want to see children's participation in research increase, in order to find more cures and better therapies. However, expanding children’s participation in research will likely require a substantial increase in funding from the federal government, non-profit groups and private industry.
Researchers should keep in mind several important factors involving children in research, from parents' point of view. Safety is unquestionably the most important factor in parents’ minds. Also, many parents are reluctant to have their children participate in research if they perceive they will be “guinea pigs". Large numbers of parents remain unsure about having their children involved in research. Parents may be more willing to have their children participate if they better understand the systems put in place to help ensure that studies involving children are safe. Doctors play an important role and can help parents understand these systems and weigh the risks and benefits of participating in research.



Data Source & Methods
This report presents findings from a nationally representative household survey conducted exclusively by Knowledge Networks, Inc, for C.S. Mott Children’s Hospital via a method used in many published studies. The survey was administered from Dec 6, 2007 - Jan 4, 2008, to a randomly selected, stratified group of adults aged 18 and older (n=2,131) with and without children from the Knowledge Networks standing panel that closely resembles the U.S. population. The sample was subsequently weighted to reflect population figures from the Census Bureau. The survey completion rate was 64% among panel members contacted to participate.
This Report includes research findings from the C.S. Mott Children's National Poll on Children's Health, which do not represent the opinions of the investigators or the opinions of the University of Michigan. The University of Michigan reserves all rights over this material.
Citation
Davis MM, Singer DC, Butchart AT, Clark SJ. Children in research: Many parents willing if risk of harm is small. C.S. Mott Children’s Hospital National Poll on Children’s Health, University of Michigan. Vol 3, Issue 5, May 2008. Available at: http://mottpoll.org/reports-surveys/children-research-many-parents-willing-if-risk-harm-small.
